A Level Biology Project
Aims |
This is an experiment to examine how the population of yeast alters over a number of days.
- Aims
- Background Information
- Aparatus
- Method
- Prediction
- Results
- Interpretation
- Limitations
- Anomolies
- Extension Work
Background Information |
Yeast is a single celled fungus. It reproduces asexually by budding. It respires anaerobically.
Formula: Yeast + Carbohydrate ------------> Alcohol + Carbon Dioxide
Apparatus Needed For The Experiments |
- Yeast
- Cider
- Conical Flask
- Muslin
- Haemocytometer
- Colorimeter
- Pipette
- Pipette Filler
- Measuring Flask
Method |
I am planning to use two methods to measure how the population of yeast changes over time. Method 1 uses a haemocytometer whilst method 2 uses a colorimeter to measure the number of yeast cells each day.
A haemocytometer is a microscope slide which has an etched grid on it. It consists of a 1mm² square known as a A square which is divided into 25 B squares which have an area of 0.04mm². These B squares are each divided into 16 C squares which have an area of 0.0025mm².
A colorimeter is a machine that is used to see how much light can pass through a liquid. It shows how much light is being transmitted through a sample of liquid. As the number of cells gets higher, less light will be transmitted through the sample. Special thin walled test tubes are used in the colorimeter so that they do not affect the amount of light passing through the sample.
To prepare the yeast solution do the following steps.
1. Measure out 50cm³ of cider into a conical flask using a measuring flask. Cider is used to provide food for the yeast to grow in.
2. Using a 1cm³ pipette carefully measure out 1mm³ of yeast suspension and add it to the conical flask containing the cider.
3. Mix the solution and then put a muslin cloth over the top of the conical flask which should be held in place using an elastic band.
4. Put this conical flask in an oven pre heated to 40ºC. The change in yeast population can then be measured by using a haemocytometer and by using a colorimeter.
The Haemocytometer Test
1. First set up the microscope, place the cover slip on the haemocytometer slide between the two grooves and put the slide on the microscope.
2. A drop of yeast solution should be taken from the conical flask using a dropper pipette and put next to the cover slip. The yeast solution will be drawn under the cover slip by capillary reaction.
3. Focus the microscope so that you can see the smallest type C squares.
4. Count the number of yeast cells that you can see in 10 randomly picked squares. Note down all these results in a table such as the one below.
| Test Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Average |
| Day 1 | |||||||||||
| Day 2 | |||||||||||
| Day 3 | |||||||||||
| Day 4 | |||||||||||
| Day 5 | |||||||||||
| Day 6 |
This haemocytometer test should be repeated daily for about two weeks. The results in the table can then be plotted into a graph.
The Colorimeter Test
The number of cells can be measured by with a colorimeter using the following method.
1. Calibrate the colorimeter by pouring 3cm³ of plain cider into a colorimeter test tube and placing the test tube into the colorimeter. Adjust the colorimeter so that with the plain cider it is showing 100% light transmission. Take the plain cider test tube out of the machine.
2. Take the conical flask containing the yeast solution out of the oven. Using a pipette measure out 3cm³ of the yeast solution into a clean colorimeter test tube.
3. Place this test tube into the colorimeter into the machine and note down the value of light transmission into a table such as the one below.
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Colorimeter Reading |
4. Pour the yeast solution back into the conical flask. Put the muslin back on the flask and return it to the 40ºC oven.
Both the haemocytometer and the colorimeter method of measuring the change in yeast cell population should be repeated at daily intervals for roughly two weeks.
A graph of Cell Numbers against time can then be plotted using the data. To convert the colorimeter readings to numbers of cells a calibration chart should be used.
Predictions |
I predict that as the number of days goes up the number of yeast cells in the solution will go up. This will continue for a number of days until the rate starts to slow down and eventually it will stop going up as no new cells are being produced.
The rate will go up over time because the yeast cells reproduce asexually which means that they are able to reproduce very quickly. They also have the perfect conditions for reproducing. They are being kept at a temperature of 40ºC which is very near to their optimum living temperature. They have lots of food from the cider and they have a lot of space to reproduce in. The rate will slow down after a while because the yeast will start killing itself off with the alcohol which it produces as part of it's respiratory process. This is known as a negative feedback reaction.
Results |
The Experiments - started to carry out the above experiments but after a few days decided to give up on the haemocytometer test because it was too time consuming. I carried out the colorimeter readings as written above and the results can be seen below. In order to get the number of yeast cells produced I am using a calibration curve so that by using the percentage transmission results the number of cells per mm² can be read off it.
I carried out the above experiment and these results were obtained. To make the colorimeter test as accurate as possible as well as my own results I used six other peoples sets of results and then took the average of each days results to work out how many yeast cells there are per mm² using the calibration curve.
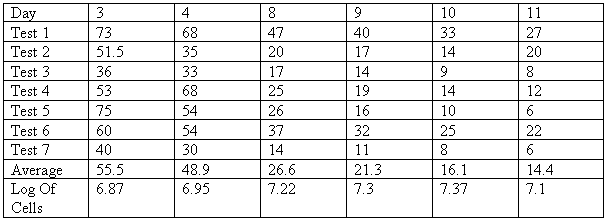
A graph of the log of the cell numbers against time was plotted. A log graph is used because the increase in the number of cells per mm² over the 11 days is so big that it would not be practical to have a graph with numbers that large on it. This graph shows the total cell numbers both living and dead.
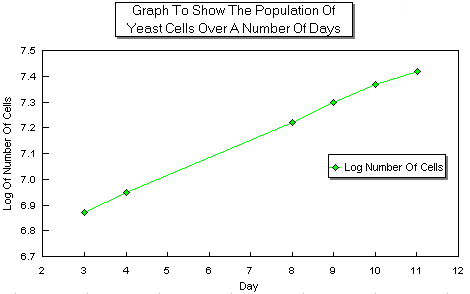
Interpretation |
The number of cells increased as the time went by as I had predicted. It increased as a fairly steady rate which can be seen on the Log graph. The population is able to increase at this rapid rate due to it's asexual reproduction. When the yeast cell is ready to reproduce a bud starts to grow out from it’s cell membrane which gets bigger and bigger until it is big enough to break off and become a new yeast cell. This is shown in the diagram below. [not reproduced]
These two yeast cells are then able to reproduce making four cells then eight then sixteen and so on. The yeast cells have plenty of space to grow in and lots of food from the cider. They are also being kept at 40ºC which is close to their optimum temperature for living. The number of cells does not keep doubling in this way. This is because the cells start to die through natural death and they are also killed by the alcohol which the yeast produce as part of their natural respiration process.
From day 3 to 4 the Log number increases from 6.87 to 6.95 which is an increase in cell numbers from 7413102 to 8912509. This is an increase of 20% for one day. From days 8 to 9 the Log goes up from 7.22 to 7.30 which is an increase in cell numbers from 16595869 to 19952623 which is an increase of 20% for one day. This shows that the rate of increase up to day nine is very steady.
After day nine the rate of population increase starts to slow down which is shown by the decreasing gradient on the graph. From day 10 to 11 the Log goes up from 7.37 to 7.41 which shows an increase in cell numbers from 23442288 to 25703958 which is an increase of 9.6% for one day. This is a fairly large drop in the expansion of the population of yeast. The rate of increase from day 10 to 11 is half that of the increase from day 3 to 4.
The rate has started to decrease by 9 and 10 because the yeast is starting to be killed off by the alcohol. This alcohol is produced as a by-product to the yeast's respiring. The alcohol poisons the yeast which causes it to die. This is known as a negative feedback reaction. The increase in yeast cells numbers leads to less space for them to reproduce so they are having to compete for space. The cells which don't have enough space will soon die. The yeast cells may also be running out of carbohydrates from the cider so this could lead them to die. The increase in alcohol and the lack of space starts to kill the cells off and so after 9 to 10 days the rate of increase in cell numbers slows down. If the experiment was continued for a few more days then I would expect the rate to stop as by then all the yeast cells would be dead.
The graph plotted of cell numbers does refer to the total number of cells both living and dead. If just the number of living cells was counted then the number of cells would start by rising at a slow rate as reproduction is only just beginning (Lag Phase). The numbers of yeast cells would then rise at a very fast rate before levelling off (Log Phase). By the time C is reached the alcohol levels have built up so the birth rate is equal to the death rate causing the population to remain constant. After D the numbers would then start dropping as the yeast cells die from lack of space and alcohol poisoning. This is known as the death phase. The theoretical curve is shown below. [not reproduced]
A - B Lag Phase
B - C Log Phase
C - D Stationary
D - E Death Phase
Limitations |
To help make this experiment more accurate, I used seven sets of results and then used the average of all the results to plot a graph with a line of best fit. I tried to keep all the variables the same for all the experiments. However, in reality it is impossible to keep all the variables precisely the same. For example:
a) It is also impossible to precisely measure out the amounts of Yeast or cider when making the solution. As the scale on the pipettes shows the volume to the nearest mm³ the volume of the solutions that I used should be correct to the nearest mm³.
b) It is also impossible to perfectly calibrate the colorimeter corectly each time. This slight variation could lead to some minor innacuracies in the results. The colorimeter was used because the haemocytometer method was taking too long to do.
c) The experiment was not carried out at the same time each day which could lead to uneven gaps between measurements.
d) The oven temperature may not have remained constant all the time causing the rate of reproduction to alter as the oven temperature changed.
Anomolies |
The plotted results on the graph produce a straight line of best fit to begin with which then goes into a curve of slightly decreasing gradient. It is a very smooth graph so no anomalies are present.
Extension Work |
This experiment could be improved in a number of ways. It could be repeated more times to help get rid of any anomalies. A better overall result would be obtained by repeating the experiment more times because any errors in one experiment should be compensated for by the other experiments.
The test should be carried out at the same time each day to ensure that the length of time between each measurement is the same.
A test that could differentiate between living and dead cells would be very useful because then just the numbers of living cells could be counted. This would tell us how the population of living cells alters over time rather than at the moment where we can only find out how the total number of cells changed over time.
Different sources of carbohydrate apart from cider could be used to see if this affects the rate that the yeast numbers increase.
Different sized flasks could be used as this could increase or decrease competition between the yeast cells depending on the size. This would tell us how big a factor the competition between the cells is.
DisclaimerThis is a real A-level school project and as such is intended for educational or research purposes only. Extracts of this project must not be included in any projects that you submit for marking. Doing this could lead to being disqualified from all the subjects that you are taking. You have been warned. If you want more help with doing your biology practicals then have a look at 'Advanced Level Practical Work for Biology' by Sally Morgan. If you want more detailed biology information then I'd recommend the book 'Advanced Biology' by M. Kent. |
